Gene Therapy for HCM
The role of genetics
To understand gene therapy as a potential treatment for HCM, it helps to know some basic information about genetics. To see how HCM is passed down in families click here.
Cells are the basic building blocks of all living things.1 They contain many different parts, including a person’s genetic information.
Cells
The human body is made up of trillions of cells. Cells contain many parts including chromosomes.
Chromosomes
Chromosomes are long strands of DNA.2

DNA
Within DNA are genes.
Gene (section of DNA)
Genes contain the instructions to make proteins.

Proteins
Proteins work together to tell your body how to grow, develop, and function.3,4 For example, proteins are involved with making your organs—including your liver, lungs, and heart—work.4
How changes in a gene can cause genetic conditions
Mutations (also known as variations or changes) in a gene can affect that gene’s ability to make a given protein the way it should or can affect whether that protein is made at all. In some cases, too much or too little of a necessary protein can result in a disease or condition.
In genetic HCM, mutations can prevent heart muscle cells from making enough protein to enable the heart to pump as expected. The most common cause of HCM is due to mutations in the MYBPC3 gene.5 For more information about the genetic causes of HCM, click here.
What is gene therapy?
Gene therapy is a way of treating or preventing diseases or medical conditions caused by genetic mutations. Gene therapy delivers a working copy of a gene into a cell to help the cell build the necessary protein and restore normal function.6
There are several types of gene therapy and each one uses a different approach to address disease-causing mutations.7 For example:
Gene Replacement Therapy
- Gene replacement therapy adds a working gene to supplement or replace the function of a non-working gene to address the underlying cause of a condition. It is also called gene addition because this approach adds a working gene into target cells.8
- Think of this as delivering a new set of “genetic instructions” into a person’s cells. This can be done by using a capsid (also called a vector) as a vehicle to deliver a working gene into cells.
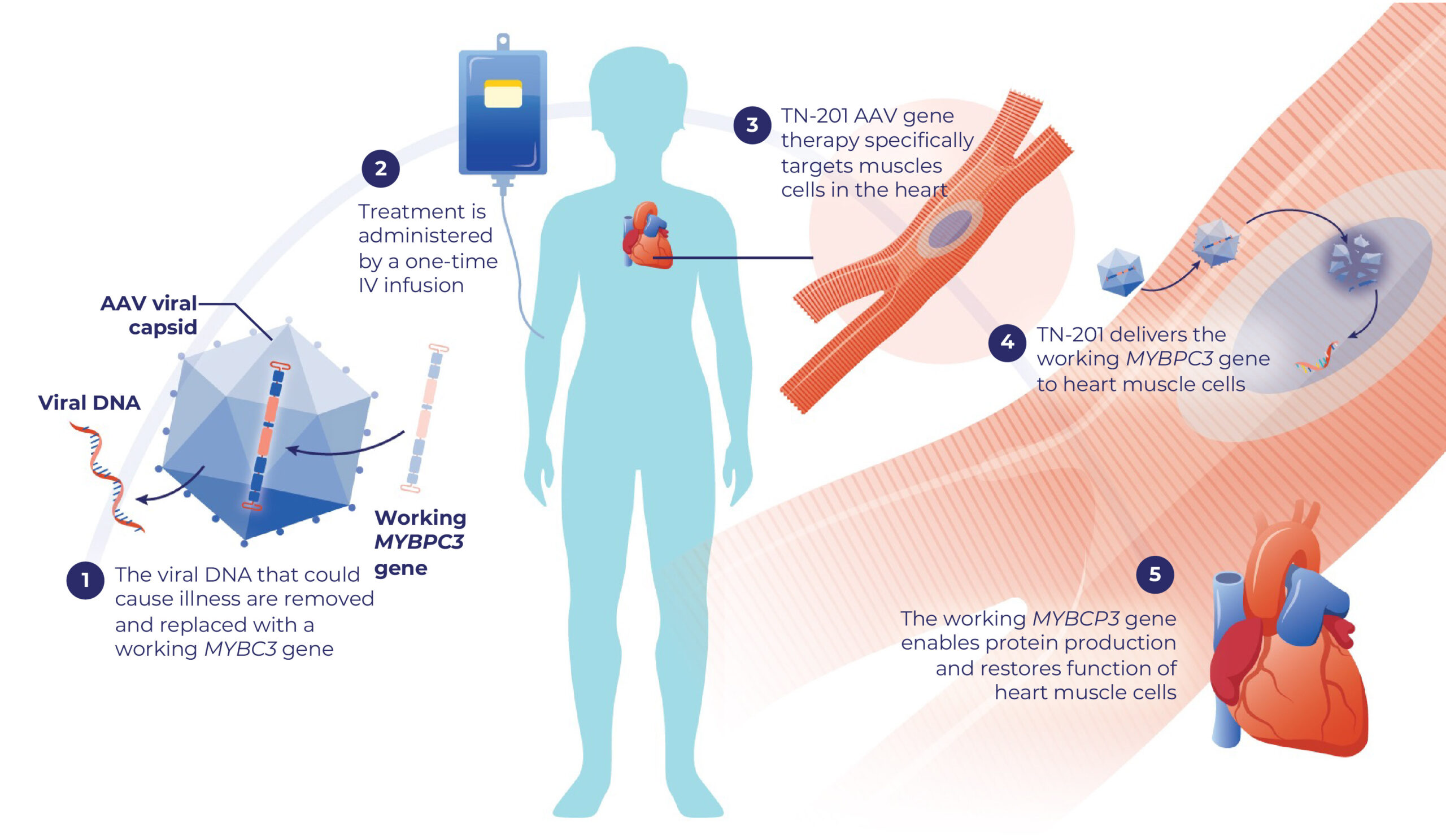
- Tenaya’s investigational treatment for HCM is a gene replacement therapy called TN-201 designed to deliver a working MYBPC3 gene to specific muscle cells that make the heart contract and relax with each beat.
Gene Editing
- Gene editing makes changes in the DNA to correct (or edit) faulty information caused by a mutation.8
- Think of this as rewriting the “genetic instructions” within a person’s DNA. Gene editing may be used when a “misspelling” of a gene results in a genetic condition. The genetic misspelling (or mutation) is corrected to address the root cause of the genetic condition.9
- Gene editing is a promising approach to addressing the precise underlying cause of disease, but researchers are still learning about its potential in genetic heart conditions.
Why are AAV capsids used in gene therapy?
- It is efficient at delivering new genes to cells, and
- There are different types of AAVs that can be tailored to target specific types of cells (such as heart muscle cells)
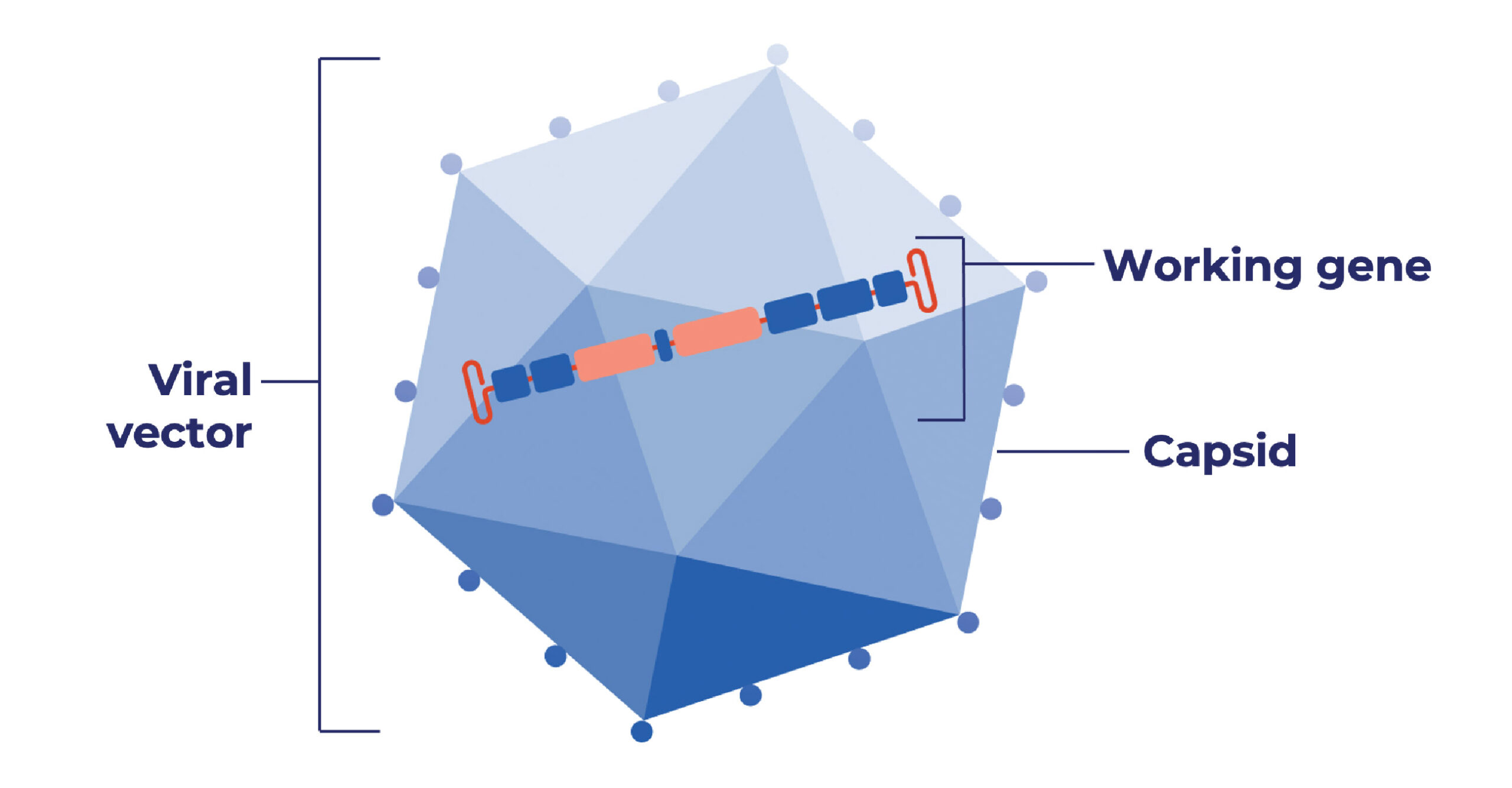
- Removes all viral genes that could cause disease or illness, leaving the capsid
- Adds a working gene to specific cells
- Does not change or edit a person’s own DNA
- Does not make changes to a person’s sperm or egg cells (and therefore, the gene therapy cannot be passed from a parent to a child)
- Will not prevent or lower a person’s risk of passing a genetic condition to their children
For more than 20 years, AAV gene therapies have been studied in thousands of people for the treatment of many different genetic conditions.12-16 Both the U.S. Food and Drug Administration and the European Medicines Agency have approved AAV gene therapies to treat several genetic conditions.17-21 Research on AAV gene therapies continues to assess how they can be used to treat more genetic conditions like HCM.
As of November 2024,
The U.S. FDA has approved six AAV gene therapies17:
- A treatment for people with inherited retinal disease (IRD)
- A treatment for children less than two years old with spinal muscular atrophy (SMA)
- A treatment for adults with hemophilia B
- A treatment for children ages four to five with Duchenne muscular dystrophy (DMD)
- A treatment for adults with severe hemophilia A
- A treatment for adults and children with aromatic L-amino acid decarboxylase (AADC) deficiency
The European Commission has authorized approval in the European Union for three AAV gene therapies:
- A treatment for adults with severe hemophilia A18
- A treatment for adults and children aged 18 months and older with severe aromatic L-amino acid decarboxylase (AADC) deficiency19
- A treatment for children with spinal muscular atrophy (SMA)20
- A treatment for an inherited retinal (eye) disease (IRD) that causes blindness21
About Tenaya’s TN-201 gene therapy for MYBPC3-associated HCM
TN-201 is an investigational gene therapy designed to reach the specific muscle cells that make the heart contract and relax with each beat.
- TN-201 uses a specific type of AAV capsid called AAV9.
- AAV9 capsids have been used to treat thousands of people around the world with gene therapy. The AAV9 capsids have been extensively studied in thousands more in clinical trials, including for heart disease.10,12
- The AAV9 capsid in TN-201 delivers a working MYBPC3 gene to the muscle cells in the heart
- Once in these cells, TN-201 may help make the protein needed to restore typical heart function so the heart can pump as expected
- TN-201 is given as a one-time intravenous (IV) infusion
How TN-201 Works
This brochure will help you understand how Tenaya’s gene therapy may help heart cells make protein.
Learn more about the clinical trial for TN-201 in people with MYBPC3-associated HCM.
From Gene to Protein
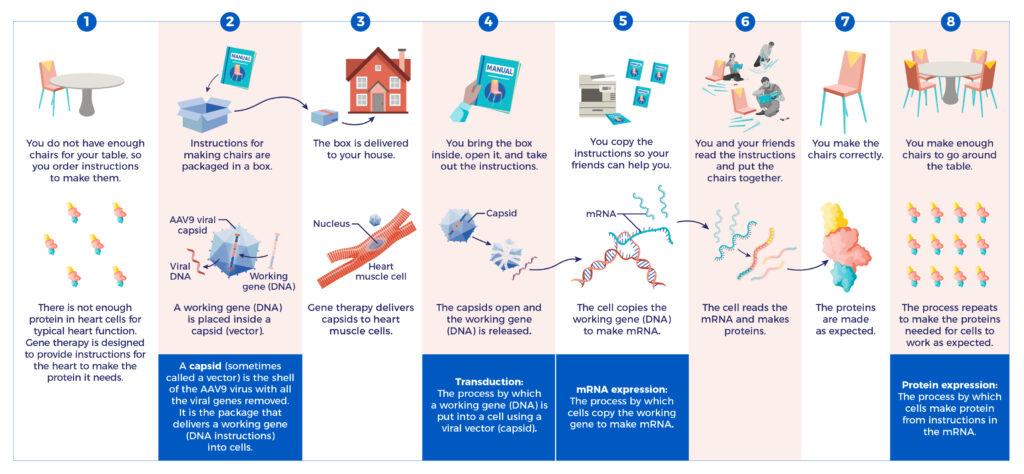
Tenaya’s gene therapies are investigational and have not been approved by the U.S. Food and Drug Administration (FDA) or any other country’s health authority or regulatory agency.
Expand for References
1. Studying cells. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of General Medical Sciences; 2023. https://www.nigms.nih.gov/education/Pages/Glossary.aspx#letter-C. Accessed April 15, 2024. 2. Glossary. American Society of Gene + Cell Therapy; 2023. https://asgct.org/education/more-resources/glossary. Accessed April 15, 2024. 3. Gene. U.S. Department of Health and Human Services, National Institutes of Health, National Human Genome Research Institute; 2024. https://www.genome.gov/genetics-glossary/Gene. Accessed April 15, 2024. 4. Protein. U.S. Department of Health and Human Services, National Institutes of Health, National Human Genome Research Institute; 2024. https://www.genome.gov/genetics-glossary/Protein. Accessed April 15, 2024. 5. van Velzen HG, Schinkel AFL, Oldenburg RA, et al. clinical characteristics and long-term outcome of hypertrophic cardiomyopathy in individuals with a MYBPC3 (Myosin-Binding Protein C) founder mutation. Circ Cardiovasc Genet. 2017;10(4):e001660. https://doi.org/10.1161/CIRCGENETICS.116.001660. 6. Gene Therapy. Department of Health and Human Services, National Institutes of Health, National Human Genome Research Institute; 2024. https://www.genome.gov/genetics-glossary/Gene-Therapy. Accessed April 15, 2024. 7. Vectors 101. American Society of Gene + Cell Therapy. 2024. https://patienteducation.asgct.org/gene-therapy-101/vectors-101. Accessed April 15, 2024. 8. Gene Therapy Approaches. American Society of Gene & Cell Therapy; 2023. https://patienteducation.asgct.org/gene-therapy-101/gene-therapy-approaches. Accessed April 15, 2024. 9. Gene Editing. American Society of Gene + Cell Therapy. 2021. https://patienteducation.asgct.org/gene-therapy-101/gene-editing. Accessed April 15, 2024. 10. Li C, Samulski RJ. Engineering adeno-associated virus vectors for gene therapy. Nat Rev Genet. 2020;21(4):255-272. https://doi.org/10.1038/s41576-019-0205-4. 11. Naso MF, Tomkowicz B, Perry WL 3rd, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 2017;31(4):317-334. https://doi.org/10.1007/s40259-017-0234-5. 12. Kuzmin, DA, Shutova MV, Johnston NR, et al. The clinical landscape for AAV gene therapies. Nat Rev Drug 2021;20(3):173-174. https://doi.org/10.1038/d41573-021-00017-7. 13. Au HKE, Isalan M, Mielcarek M. Gene therapy advances: a meta-analysis of AAV usage in clinical settings. Front Med (Lausanne). 2022;8:809118. https://doi.org/10.3389/fmed.2021.809118. 14. George LA, Ragni MV, Rasko JEJ, et al. Long-term follow-up of the first in human intravascular delivery of AAV for gene transfer: AAV2-HFIX16 for severe hemophilia b. Mol Ther. 2020;28(9):2073-2082. https://doi.org/10.1016/j.ymthe.2020.06.001. 15. Novartis. Q3 2022 Results; 2022. Accessed March 29, 2023. 16. Keeler AM, Flotte TR. Recombinant adeno-associated virus gene therapy in light of Luxturna (and Zolgensma and Glybera): where are we, and how did we get here? Annu Rev Virol. 2019;6(1):601-621. https://doi.org/10.1146/annurev-virology-092818-015530. 17. S. Food and Drug Administration (FDA); 2024. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products. Accessed April 15, 2024. 18. European Medicines Agency; 2023.https://www.ema.europa.eu/en/medicines/human/EPAR/roctavian . Accessed April 15, 2024. 19. European Medicines Agency; 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/upstaza. Accessed April 15, 2024. 20. European Medicines Agency; 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/zolgensma. Accessed April 15, 2024. 21. European Medicines Agency; 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/luxturna. Accessed April 15, 2024.
